PRODUCT IDENTIFICATION
CLASSIFICATION
NAPHTHOLS /
PHYSICAL AND CHEMICAL PROPERTIES
Light creamy beige powder
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
Light creamy beigr powder
98.0% min
MELTING POINT
246 C
INSOLUBLES
0.1% max
BON ACID
0.1% max
|
|
|
| NAPHTHOL AS | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 92-77-3 |
|
| EINECS NO. | 202-188-1 | |
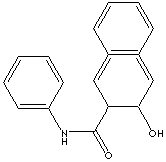
| FORMULA | C17H13NO2 | |
| MOL WT. | 263.30 | |
| H.S. CODE | ||
| SMILES |
| |
| TOXICITY | ||
| SYNONYMS | 3-Hydroxy-2-Naphthoic Aicd Anilide; C.I. 37505; | |
| 3-Hydroxy-N-phenyl- 2-Naphthalenecarboxamide; C.I. Azoic Coupling Component No. 4; Naphthol AS-BO; | ||
|
CLASSIFICATION |
NAPHTHOLS / |
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
Light creamy beige powder | |
| MELTING POINT | 247 C | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| AUTOIGNITION | ||
| pH | ||
| VAPOR DENSITY | ||
| NFPA RATINGS | ||
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
| Naphthols are either of two crystalline monohydric alcohols, derived from naphthalene and belong to the phenol family. There are position isomers; alpha-naphthol (also called 1-naphthol) is 1-hydroxynaphthalene and beta-naphthol (also called 2-naphthol) is 2-hydroxynaphthalene. The compound 1-naphthol is made by heating 1-naphthalenesulfonic acid with caustic alkali or by heating 1-naphthylamine with water under pressure. The compound 2-naphthol is manufactured by fusing 2-naphthalenesulfonic acid with caustic soda. The different positions provide various chemical structures which offer important roles to each characteristic colours. Naphthols are slightly soluble in water but readily soluble in short alcohols, ethers chlorinated solvents, and caustic alkalis. They are used directly in making several dyes and are converted into numerous corresponding amines, esters, ethers and carboxylic derivatives as well as into numerous sulfo- and nitro-group substituted (mono-, di- and tri) naphthol compounds. They find extensive applications in making dyes, pigments, fluorescent whiteners, tanning agents, antioxidants, and antiseptics. Naphthol structure is found as a ligand in transition-metal catalysts particularly in the form of binaphthol (binol) which is composed of two naphthol rings connected at one carbon site on each ring. Optically active binol is widely used in asymmetric synthesis of rearrangements, epoxidations, reductions. | ||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Light creamy beigr powder | |
| PURITY |
98.0% min | |
|
MELTING POINT |
246 C | |
|
INSOLUBLES |
0.1% max | |
|
BON ACID |
0.1% max | |
| TRANSPORTATION | ||
| PACKING | 25kgs in bag, 1mts in bag | |
| HAZARD CLASS | ||
| UN NO. |
| |
| OTHER INFORMATION | ||
| Hazard Symbols: XI, Risk Phrases: 36/37/38, Safety Phrases: 24/25 | ||
|
|
|